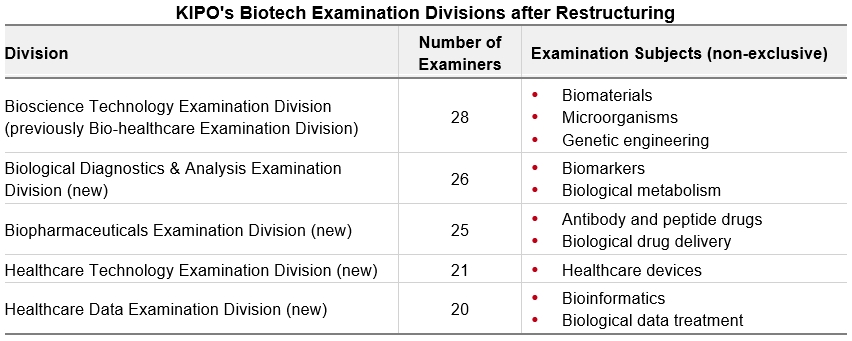
On March 10, 2025, the Korean Intellectual Property Office (KIPO) announced a restructuring of its examination bureau designed to increase efficiency and expertise in examination for biotech inventions. The restructuring replaces the current biotech examination division with five specialized biotech divisions: the Bioscience Technology Examination Division, Biological Diagnostics & Analysis Examination Division, Biopharmaceuticals Examination Division, Healthcare Technology Examination Division, and Healthcare Data Examination Division. These five divisions comprise a total of 120 examiners, including 35 examiners newly hired in February 2025.
The restructuring continues KIPO's recent efforts to expedite examination of biotech patents. In February 2025, KIPO announced that it added biotech applications to the scope of "advanced technology" patent applications eligible for expedited examination (see our legal update article of February 26, 2025 (Link)). Previously, "advanced technology" was limited to covering semiconductor, display, and secondary battery technologies, but KIPO has now revised the relevant regulations to add biotechnology (as well as AI and advanced robotics) to this list. Applicants for biotech inventions (and other "advanced technology" applications) can request expedited examination of their applications as long as they are engaged in the production or preparation of biotech products or devices in Korea, or if the invention is the result of a national research and development project. Expedited examination can significantly reduce the amount of time it takes to receive a patent, since applicants can obtain patents after as little as 2 months of examination, instead of the average 18.9 months taken for regular non-expedited examination in Korea.
These changes confirm the Korean government's strong emphasis on supporting the biotech industry in Korea. The Government has designated biotech as one of three "economic driving forces" (together with AI/semiconductor and quantum technologies), and launched the National Bio Committee in January 2025 to discuss strategies and policies for further fostering the biotechnology industry in Korea. These efforts seem to be reflected in the fact that in the past five years, the number of patent applications for biotech inventions has increased by an average of 8.2% per year, an increase about 3.5 times greater than the average increase in all technical fields combined (2.3%).
Korea is expected to continue to see rapid growth in the biotechnical field, based on both substantial private investment and governmental support. KIPO's efforts to increase the number of examiners reviewing such applications, and to bolster examination expertise in specialized areas of biotech, are expected to significantly benefit biotech patent applicants by increasing the efficiency and quality of review of such applications in the future.
Related Topics






