On February 21, 2025, the Ministry of Food and Drug Safety ("MFDS") implemented a new system for protecting clinical data submitted for an original drug's marketing authorization ("MA"), replacing the previous drug re-examination system (which only provided de facto data exclusivity).
On the same day, the MFDS also amended the Regulation on Safety of Pharmaceuticals, etc. to identify specific categories of drugs that are subject to the data protection system as delegated by the Pharmaceutical Affairs Law, and published a Q&A booklet with details regarding the new system.
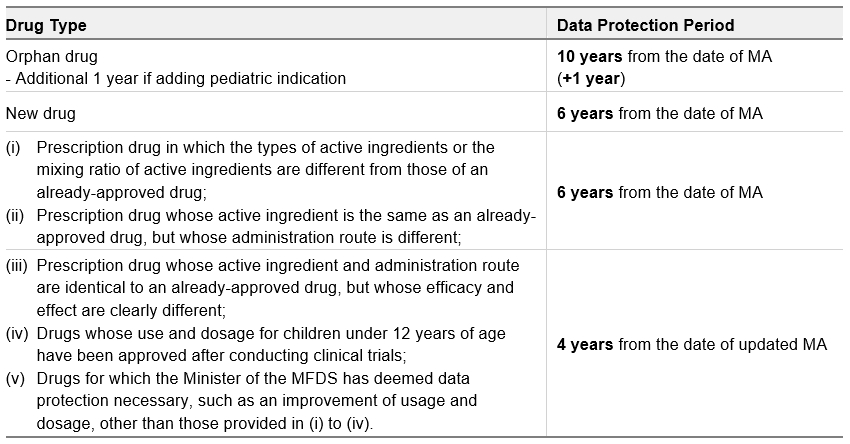
In the Q&A booklet, the MFDS clarifies that the new system applies to (i) drugs for which an MA is issued on or after February 21, 2025, and (ii) original drugs that have orphan drug status as of February 21, 2025, or new drugs approved less than six years before February 21, 2025. This means that original orphan drugs that received an MA after February 21, 2015 are now protected under the new system until the 10th anniversary of their MA date (regardless of their re-examination status under the old system). Any entity with an original orphan drug approved in Korea is advised to check the data protection period published on the MFDS website (in Korean).
The periods of data protection under the new system can be summarized as follows:
We will continue to monitor the implementation of the new system and provide further updates as appropriate.
Related Topics






