The Korean Intellectual Property Office (KIPO) has promulgated a new Enforcement Decree for the Korean Patent Act (effective as of July 14, 2020). Changes of note introduced by the new Enforcement Decree include: (i) a reduction in the periods counted for patent term adjustment ("PTA"); and (ii) clarification that product approvals under the Narcotics Control Act are a valid basis for patent term extension ("PTE").
Changes to Patent Term Adjustment
Currently, Korea provides PTA to extend patent terms for patents whose issuance has been unduly delayed due to delays in examination by KIPO. However, the new Enforcement Decree has expanded the number of actions that KIPO will consider "delays attributable to the applicant" and thus not subject to inclusion in the PTA calculation, to improve consistency with other major jurisdictions. Under the Patent Act, PTA begins to accrue either 4 years after the filing date of the patent application or 3 years after the request for examination (whichever is later) and ends at patent registration. Under the current Enforcement Decree, any delays attributable to the applicant (typically periods indicated in orange below) are deducted from PTA.
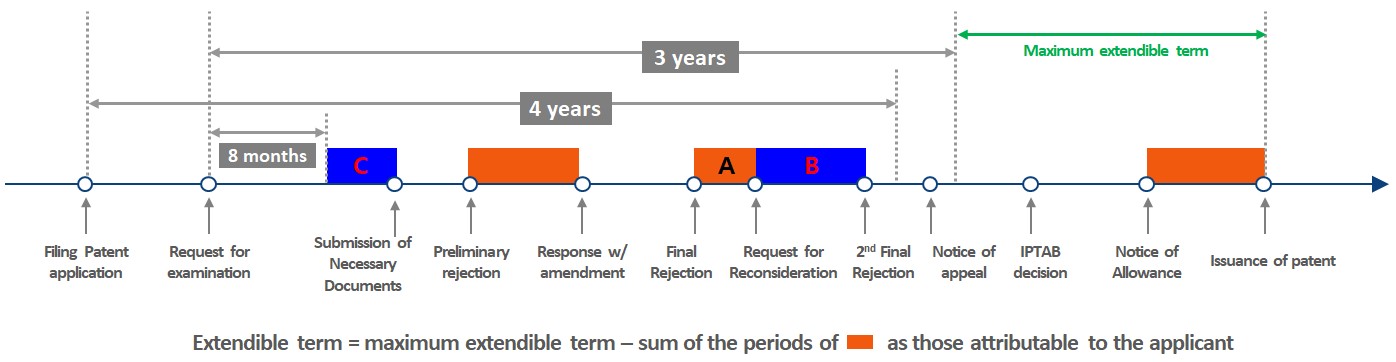
Under the new Enforcement Decree, the periods marked B and C in the above diagram (in blue) will also be considered delays attributable to the applicant to be excluded from PTA. In detail, period B extends the period of delay attributable to the applicant with respect to requesting reconsideration of a Final Rejection (previously, the applicant delay only extended to the point where the request for reconsideration was filed (period A), but now the entire reconsideration period (A+B) is considered applicant delay), and period C essentially means that if an applicant takes more than 8 months after a request for examination of an application to submit certain documents relevant to the examination, no further PTA will accrue (i.e., the delay beyond 8 months will be attributed to the applicant) until the documents are actually submitted. These documents are defined as (i) documents necessary for examination such as documents evidencing the deposit of a microorganism, applicability of the novelty grace period (exception to the novelty bar), or a priority claim, and (ii) documents submitted to correct translation errors in the Korean application.
Changes to Patent Term Extensions
The new Enforcement Decree further clarifies that product approvals under the Narcotics Control Act also are a valid basis for PTE, reflecting a Patent Court decision issued in 2019.
While the Patent Act generally provides that PTE is available for delays due to delays in obtaining permission to practice the patented invention due to required safety or efficacy testing, prior to the recent amendment, the Enforcement Decree only expressly identified pharmaceutical product approvals under the Pharmaceutical Affairs Act and agrochemical product registrations under the Pesticide Control Act as valid bases for applying for PTE. However, while the Pharmaceutical Affairs Act generally covers pharmaceutical product approvals in Korea, the Act states that drugs that are potentially addictive or subject to abuse are to be regulated and approved under a separate law, the Narcotics Control Act. As a result, KIPO and the Intellectual Property Trial and Appeal Board (IPTAB) have rejected several PTE applications based on the Narcotics Control Act on the ground that product approvals under the Narcotics Control Act were not expressly identified as a valid basis for PTE in the Enforcement Decree.
However, in 2019 the Patent Court reversed one such IPTAB decision and held that the previous Enforcement Decree improperly failed to implement the full scope of the Patent Act with respect to PTE eligibility. The Patent Court also stated that treating approvals under the Narcotics Control Act and the Pharmaceutical Affairs Act differently would be a violation of the principle of equality under the Constitution.
The new Enforcement Decree now expressly provides that product approvals under the Narcotics Control Act also are eligible for PTE. This change is an important step in improving the Korean PTE system to more adequately protect patentees' rights and better harmonize with other major PTE jurisdictions.
Related Topics





