The Patent Term Adjustment (PTA) system in Korea applies only to Korean patent applications with an effective filing date on or after March 15, 2012. While there have only been a handful of PTAs granted since the PTA system was introduced, one recently granted PTA for a pharmaceutical patent provides some insight on how PTAs are determined and granted in Korea.
Requirements for PTA Eligibility
PTA is available for a patent for the period beginning at the later of 4 years after the application filing date, or 3 years after the request for examination date, and ending when the patent is registered. For a Korean national phase filing from a PCT application, these timelines are counted from the date of the Korean national phase filing, not the international filing date. An important difference between the Korean PTA system and the system in the U.S. is that the U.S. Patent and Trademark Office automatically calculates the applicable PTA and includes it in the Notice of Allowance, whereas KIPO does not take any action to review or calculate PTA until the patentee files a PTA request.
The Patent at Issue
The patent relates to Perjeta®, a treatment for women with early stage HER2-positive breast cancer, and claims a therapeutic combination comprising pertuzumab, trastuzumab, docetaxel and carboplatin. During prosecution, the Korean Intellectual Property Office (KIPO) rejected the claims for lack of novelty and inventiveness. The Intellectual Property Trial and Appeal Board (IPTAB) affirmed KIPO's rejections, and the applicant appealed the case to the Patent Court. The Patent Court reversed the IPTAB decision and the patent was granted.
PTA Filing and Calculation
The following figure summarizes the prosecution history of the patent.*
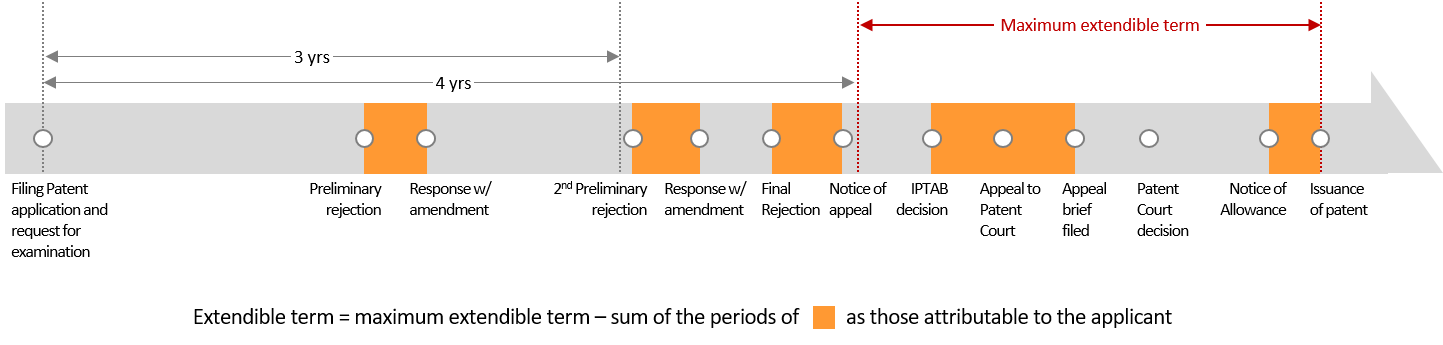
As indicated in red in the figure, the PTA term was calculated beginning 4 years after the Korean national stage filing date (which was later than 3 years after the request for examination date), with periods corresponding to "patentee delay" subtracted, resulting in a total PTA of 130 days.
Why Few PTAs Are Granted in Korea Compared to the US
While approximately 50% of prosecution cases in the US receive some amount of PTA according to the USPTO website, it is very rare for a patent prosecution in Korea to take more than 3-4 years to reach allowance, unless an appeal of a final rejection is involved, since on average it takes about 15 months after the request for examination for the first office action to issue. In fact, KIPO examiners generally will make special efforts to try to ensure examinations do not extend past the point where PTA begins to accrue. However, cases where PTA has been granted in Korea have generally involved appeals of a final rejection, and thus involved extended examination periods of between 53 to 70 months.
Maximizing Potential PTA vs PTE
Since PTA begins to accrue no earlier than 4 years after the application filing date, regardless of when the request for examination is filed, it should be noted that filing the request for examination at the same time as the application is filed will make it even less likely that the application will qualify for any PTA at all. If the request for examination is filed right away, then the substantive examination of the application must take longer than 4 years in order to qualify for PTA, whereas if the request for examination is delayed one year after the application is filed, then the substantive examination must only take 3 years before PTA begins to accrue.
Thus, if there is no urgency in obtaining the grant of the application, the applicant may consider delaying requesting substantive examination up to one year, to maximize the likelihood that the application may qualify for PTA. As a practical matter, this may not in fact result in significant delay in the examination, since the examiner would then likely be motivated to complete the examination within 3 years (after the request for examination) rather than 4 years (after the application filing).
Such a strategy may be particularly appropriate for pharmaceutical patents that will not be eligible for Patent Term Extension (PTE), such as where the approved product covered by the patent does not contain any "new chemical entity" that has not been previously approved in Korea. Of course, for patents potentially eligible for PTE, it is generally advisable to obtain allowance of the patent as soon as possible to maximize the PTE term that may be granted, rather than delaying the start of examination to maximize the chance of qualifying for PTA.
Related Topics






